Optimizing lab labels for high-performance aseptic operations: hybrid sterile + cleanroom-compatible labels
Key takeaways
- Hybrid cleanroom-sterile lab labels meet both cleanroom and sterility standards, eliminating the need for high-risk, high-cost workarounds.
- They have the highest impact in critical environments like fill/finish, cryogenic storage, and sterile device assembly.
- Cleanroom-sterile lab labels are engineered for durability after sterilization, chemical exposure, and extreme temperatures.
- They reduce compliance risk with full traceability, sterility validation, and cleanroom compatibility.
High-tech fields like medical device manufacturing and pharma depend on aseptic areas and cleanrooms that are built for precision, but many still rely on lab labels that fall short. Sterile labels may contaminate cleanrooms and cleanroom labels, even if sterilized in-house, underperform and increase regulatory burden on technicians. The result is higher risk, slower workflows, and more complicated audits.
The fix lies in dual-purpose cleanroom-sterile labels that function in all of your sensitive operational facilities. Read on to learn how and why.
Characteristics of cleanroom-sterile dual-purpose laboratory labels
Hybrid cleanroom-sterile lab labels meet the parameters for both cleanroom compatibility and aseptic standards.
- Low-particulate, low-outgassing construction
- Sterilized via validated process (gamma, EtO, or autoclave) to meet SAL requirements
- Packaged and handled to preserve both cleanliness and sterility
- Tested for post-process sterilization adhesive and print performance
Where are dual-purpose cleanroom-sterile lab labels most impactful?
Laboratory labels that are both cleanroom compatible and sterile boost efficiency in any manufacturing or research setting where products move through aseptic and cleanroom environments. Here are just a few examples where dual-purpose cleanroom-sterile lab labels could drastically reduce risk and increase productivity.
Pharmaceutical or biologic fill and finish lines
Sterile fill/finish lines represent some of the most tightly controlled environments in pharmaceutical and biologic manufacturing. These ISO 5 (Class 100) or Grade A zones where sterile drug products are dispensed into final containers — vials, syringes, or cartridges — before sealing and packaging are particularly fragile. At this stage, even the smallest source of contamination can jeopardize product integrity, delay release, or trigger a costly batch rejection.
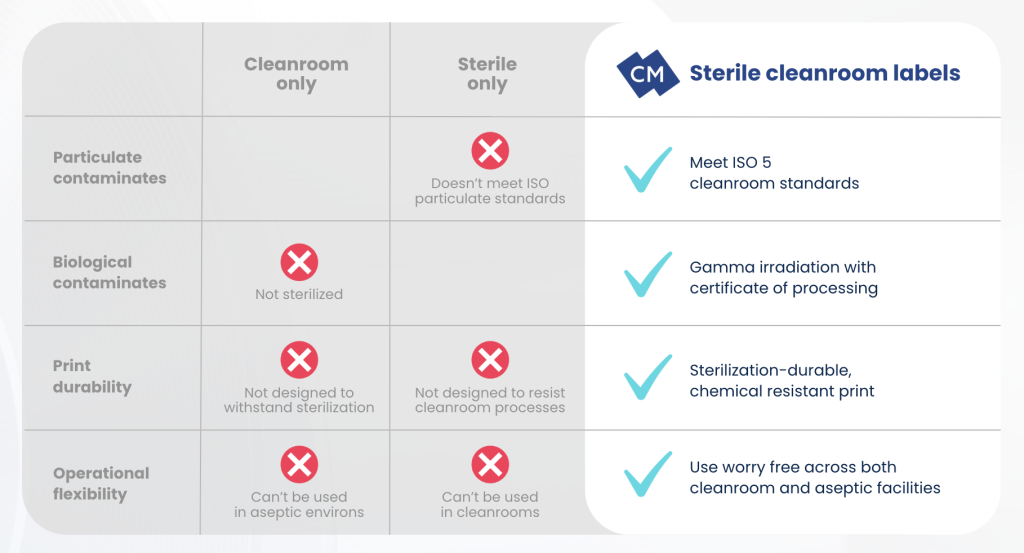
✗ Cleanroom-only lab labels
Cleanroom-only labels are typically particulate-controlled and suitable for ISO environments, but they are not sterilized and cannot be introduced into aseptic fill zones without additional validated processing.
✗ Sterile-only lab labels
Sterile-only lab labels may meet SAL standards but fail to meet particulate or static controls necessary for direct use in ISO 5 airflow or Grade A laminar hoods.
✔ Hybrid cleanroom + sterile lab labels
Pre-sterilized lab labels that are also processed in an ISO-classified cleanroom environment close this critical gap:
- They won’t shed particles, interfere with airflow, or contribute to electrostatic buildup in fill/finish isolators or RABS systems.
- They arrive with validated sterilization processes, COPs, batch-level traceability, and appropriate packaging.
- Adhesives and substrates are engineered for post-sterilization and chemical durability, maintaining adhesion, legibility, and print fidelity even after exposure to sterilization and cleanroom cleaning agents.
Biologics sample and cryopreservation workflows
Biologic therapies demand precise, sterile sample handling at every stage, including cryogenic storage. In these workflows, lab labels aren’t just identifiers. They are critical tools for maintaining traceability through temperature extremes, aseptic handling steps, and cleanroom transfer protocols.
Laboratory labels used in these applications must withstand extreme conditions: liquid nitrogen exposure, thaw cycles, surface disinfection, and sterile barrier handling — all without compromising adhesion, legibility, or particulate control.
✗ Cleanroom-only lab labels
Cleanroom-grade laboratory labels perform well in ISO-classified environments, but they are not sterilized and cannot be confidently used on primary containers holding sterile biologics or intermediates. Also, their adhesives and face stocks may fail when exposed to cryogenic conditions or disinfectants.
✗ Sterile-only lab labels
Sterile-only lab labels pass SAL requirements, but if they are not processed in a cleanroom, they risk introducing particulates or static discharge — particularly problematic when labeling cryovials, bioprocessing bags, or samples in ISO 5 transfer hoods or isolators. And, most are not validated for adhesion at cryogenic temperatures.
✔ Hybrid cleanroom + sterile lab labels
Dual cleanroom-compatible and sterile-certified laboratory labels are purpose-built for biologics workflows:
- They are processed in ISO-classified cleanrooms to prevent particulate shedding and electrostatic interference during handling.
- They are sterilized via validated processes and packaged for aseptic transfer with full traceability.
- Materials can be validated for cryogenic performance, adhering securely at -80°C or lower, maintaining legibility post-thaw, and resisting degradation from ethanol, IPA, or hydrogen peroxide-based cleanroom agents.
- They integrate seamlessly across storage, transfer, and aseptic re-entry workflows, minimizing relabeling or revalidation needs.
Medical device assembly
In medical device manufacturing, labeling often occurs during or immediately after final assembly, frequently within cleanroom or aseptic environments. Components like implantable devices, surgical kits, or diagnostic cartridges may be assembled in ISO cleanrooms, then packaged for terminal sterilization or inserted into pre-sterilized barrier packaging. At this stage, labeling is tightly regulated and must support both product integrity and compliance documentation.
Any label failure — whether particulate or sterility-related — can threaten product release, disrupt audits, or trigger recalls.
✗ Cleanroom-only lab labels
While these laboratory labels are designed for cleanroom compatibility, they do not meet sterility requirements. Applying a cleanroom-only label inside a sterile barrier package or on a product destined for terminal sterilization introduces validation gaps and contamination risk.
✗ Sterile-only lab labels
Sterile-only lab labels are not processed in ISO-controlled environments. That means they may not meet particulate or outgassing standards required for cleanroom use. They also may not withstand material stress from sterilization cycles.
✔ Hybrid cleanroom + sterile lab labels
Laboratory labels that are both cleanroom-processed and pre-sterilized bridge the operational and regulatory demands of device assembly:
- Processed in ISO-classified cleanrooms to prevent particulate shedding, ensuring compatibility with regulated cleanroom environments.
- Sterilized using validated methods with complete documentation and lot-level traceability to support device history records (DHR).
- Engineered to withstand sterilization methods without adhesive degradation, curling, or print distortion.
- Can be chemically resilient to standard cleanroom disinfectants such as IPA, bleach, or hydrogen peroxide vapor, ensuring print and adhesive integrity throughout post-assembly handling.
Key questions for your dual cleanroom-sterile lab label provider
Once you’re ready to make the switch to dual-purpose cleanroom-sterile laboratory labels, you’ll want to ask potential suppliers some key questions:
Has the product been tested for both particle and sterility standards?
Look for lab labels processed in an ISO-classified cleanroom and sterilized using a validated method like gamma irradiation. Suppliers should be able to provide particulate testing data and sterility assurance documentation.
What sterilization method is used?
Ask whether sterilization is performed in-house or through a validated partner and what method is used. The supplier should provide a certificate of processing.
Is post-sterilization print and adhesive performance validated?
Not all label materials survive sterilization intact. Ensure the supplier has tested adhesive bonding, label integrity, and print durability after exposure to sterilization conditions (e.g., temperature, humidity, radiation) and chemical resistance to typical post-process cleaning agents like IPA or hydrogen peroxide.
How is sterility preserved during packaging and transport?
Laboratory labels should be sealed in sterilization-validated packaging. Ask about expiration dates and shipping and handling procedures.
By asking these questions up front, your procurement team can ensure that laboratory labels won’t become a bottleneck — or a liability — during production, release, or audit. A strategic lab label supplier should offer more than labels. They should offer quality assurance.
CleanMark sterile cleanroom lab labels solve your operational challenges
CleanMark has a 40-year history supporting some of the most sensitive operations in the pharmaceutical, life science, and healthcare industries and has a spotless track record with our sterile and cleanroom labels. Our custom-engineered approach to lab labels means you get exactly the label you need for your unique processes, and our commitment to quality means you never have to worry about particulate or biological contamination.
Schedule a consultation to get started, or connect with one of our lab label experts now via chat. We look forward to supporting your labeling needs.
Looking for more detailed information?
Explore case studies, articles, guides and more in our extensive library of labeling resources.

The automation label: materials and construction matter

Why your automated label applicator and labels should be designed together

How automated labeling changes your label needs
Stay up to date on labeling advances in your industry
From white papers to industry surveys and ebooks, we’ll make sure you’re informed about the latest technology that can make your operations more efficient and less stressful.